Level 3 Serialization with zf-work-pharma
Independent, integrated, adaptable:
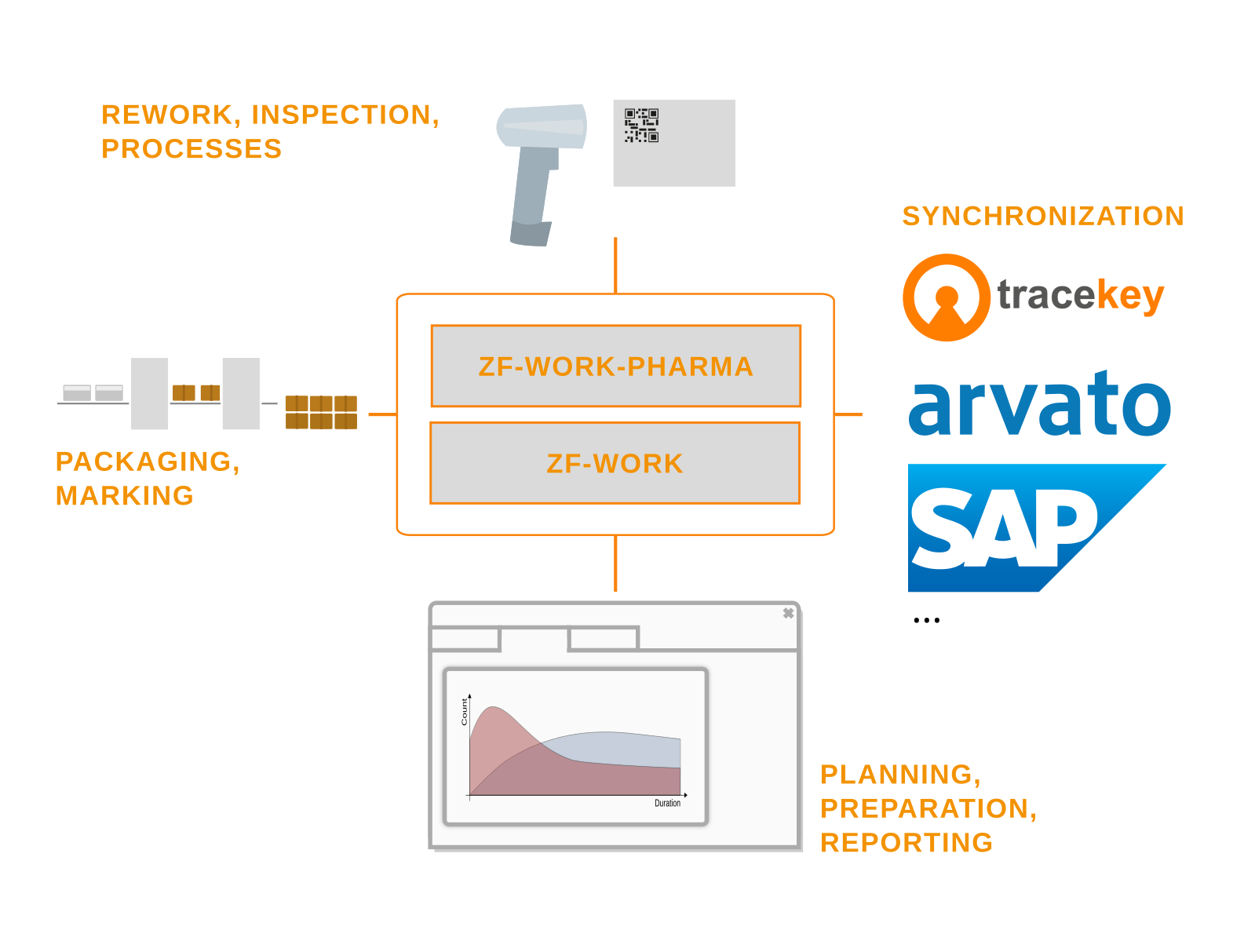
The zf-work-pharma product offers a cost-effective level 3 track-and-trace solution for pharmaceutical serialization in production. zf-work-pharma, which was developed in collaboration with tracekey solutions GmbH, perfectly complements the serialization process.
The zf-work-pharma system implements the decoupling between the remotely operated Level 4 system and the local production environment to ensure secure execution of the serialization workflow on site, independent of the remote network.
It supports integration with a standard and custom packaging systems and gives you full control over the serialization workflow from reservation over packaging, rework, and reporting, and correction if necessary.
Zf-work-pharma for Manufacturers
Because if its simple but secure operations and because of its intuitive but powerful usability, it is the reliable partner for the serialization process of manufacturers.
Zf-work-pharma for Logistics
Because of its ability to integrate, its robust workflow platform, and its first class software logistics it is the perfect track and trace workhorse for logistics companies.

Process Digitalization for Production
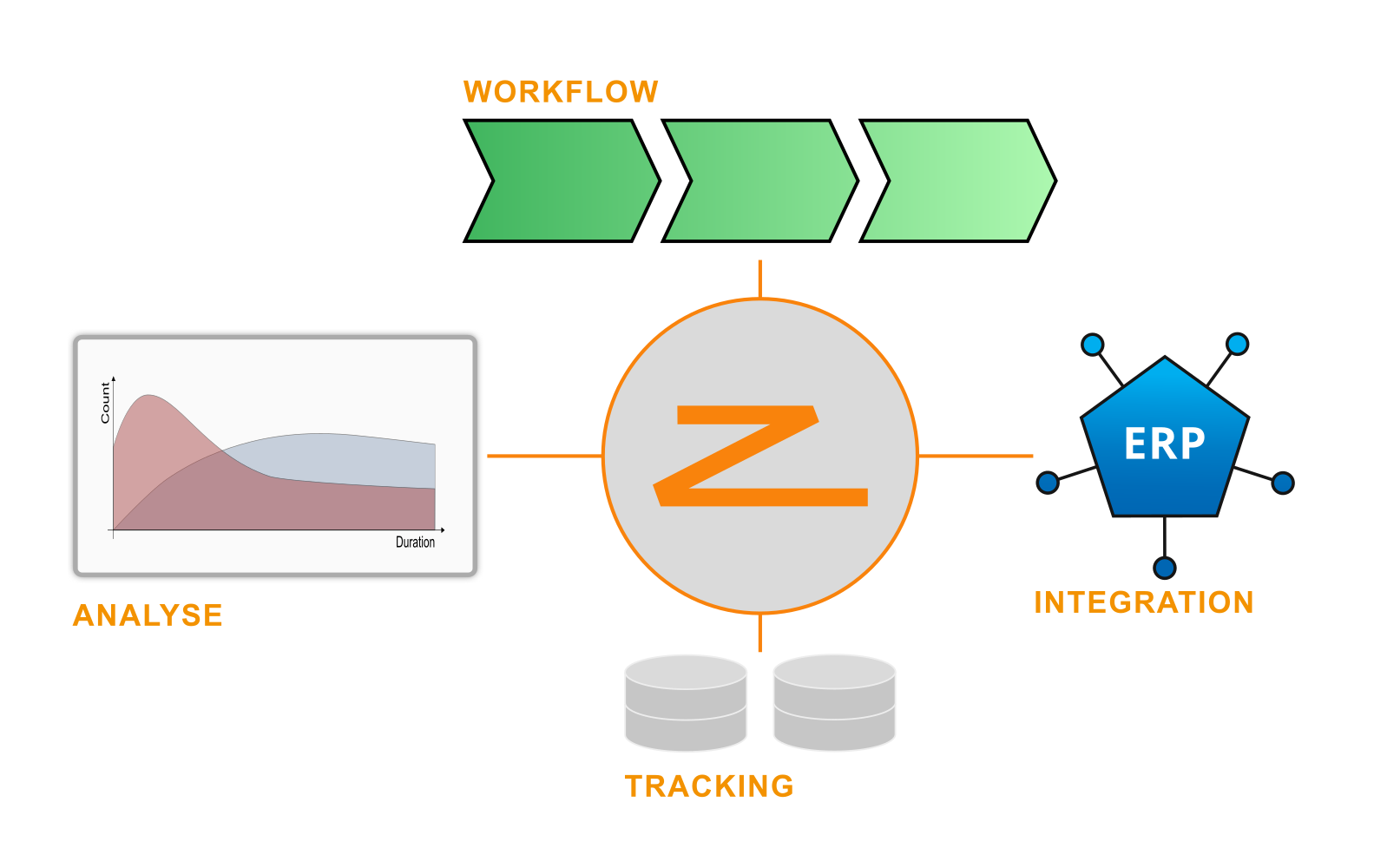
In order to record and measure production processes or correlate them with planned processes, it is essential to be able to manually record data from production at the right time and in the right place using workstations and scanners and, if necessary, machine interfaces.
There is often already a large number of data collection systems in production - whether manual or electronic. However, these are usually isolated solutions and not integrated into a comprehensive production model.
A jumble of responsibilities and systems makes an integrated view of processes difficult and makes customization and expansion according to customer requirements a nightmare.
The customer-specific mapping of production processes and the appropriate data acquisition and integration with the system involved completes the production process in its entirety with a digital image.
ZFabrik builds on tried-and-tested approaches that guarantee a high degree of flexibility in software customization and delivery transparency for the customer on the one hand and ensure high reliability and excellent comprehensibility in error situations on the other.
Essential core elements such as user-friendly data capture, robust and transparent task processing and a high degree of reuse of proven solution designs on a highly integrated software platform that is maintainable in the long term enable us to provide sustainable customer solutions.
Industrial Serialization, Track & Trace
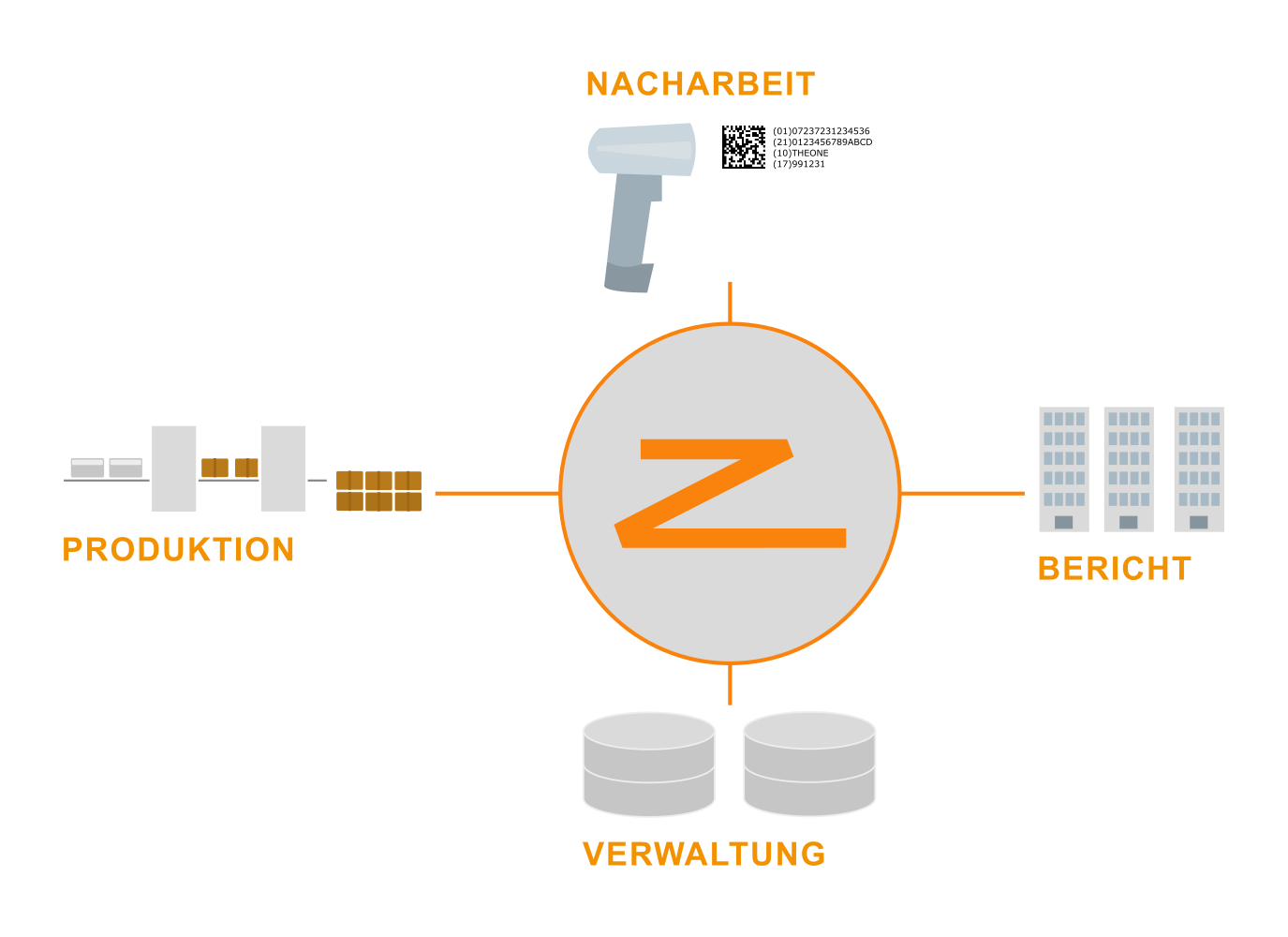
Various industries are required to implement serialization of goods due to regulatory requirements or customer requirements.
At its most simple, serialization is about getting a good serial print on a good and tracking what numbers have been applied, wasted, or kept and to eventually report on serials to customers or authorities.
Implementing serialization requires a software architecture that is capable to track millions of serial numbers and to perform complex data workflows reliably, transparently and automatically.
It must support flexible data integration with existing systems and machinery as well as customer specific extension and modification – possibly even integration into a customer run development process.
Industrial Track and Traces is more than managing serials. It is about tracking the flow of individual semi-finished goods through the production process.
Once you have implemented production tracking, you can analyze and balance your production flow without cheating: Where is the waste, where did we have the longest waiting times, when and what machine worked on this piece? And ultimately: Derive a model to understand production flow and minimize waste.
We at ZFabrik know that implementing track and trace meaningfully is not a one-off effort but rather an ongoing process and requires a strong team and an adaptable software solution that can be integrated and adapted with production systems and operator workflows.
We have been working in serialization since 2010 and implement production critical industrial serialization since 2015.
